Abstract
INTRODUCTION: Elderly patients with newly diagnosed multiple myeloma (NDMM) are highly heterogeneous and their outcome is influenced by many factors: beside age, also comorbidities, general physical fitness, and cognitive function play a crucial role. The IMWG frailty score combines age, functional status, and comorbidities, and it identifies fit, intermediate-fit and frail patients, with different risk of toxicity, treatment discontinuation, and mortality (Palumbo A et al. Blood 2015). Until now, evidence-based tailored treatments according to patients' frailty are still lacking. Therefore, this phase III study investigated the efficacy and feasibility of dose/schedule-adjusted lenalidomide-dexamethasone therapy followed by lenalidomide maintenance (Rd-R) versus continuous lenalidomide-dexamethasone (Rd) in elderly, intermediate-fit NDMM patients.
METHODS: Intermediate-fit NDMM patients, with a total frailty score (age, Charlson Index, ADL and IADL) of 1 (http://www.myelomafrailtyscorecalculator.net/), were enrolled and randomized to receive Rd-R or continuous Rd. To better approximate a real-world older population, patients usually excluded from clinical trials or with abnormal laboratory values could be included in the trial.
Rd-R treatment consisted of nine 28-day cycles of lenalidomide 25 mg/day for 21 days and dexamethasone 20 mg on days 1,8,15,22, followed by lenalidomide maintenance 10 mg/day for 21 days, until disease progression. Continuous Rd consisted of lenalidomide 25 mg/day for 21 days and dexamethasone 20 mg on days 1,8,15,22, until disease progression. The dose and schedule of continuous Rd was the one adopted in patients >75 years in the FIRST trial (Hulin C et al. JCO 2016).
The primary endpoint was event-free survival (EFS), defined as progression or death for any cause or discontinuation of lenalidomide or occurrence of any hematological grade 4 or non-hematological grade 3-4 adverse events (AEs), including Secondary Primary Malignancies (SPMs), whichever came first.
RESULTS: 199 patients (98 in Rd-R arm and 101 in continuous Rd arm) could be evaluated. Patients characteristics were well balanced between the 2 arms. Median age was 75 and 76 years (p=0.06); 47% in Rd-R vs 57% in continuous Rd were defined intermediate-fit for age (≥76 years), 53% vs 43% due to an impairment in Charlson Index, ADL or IADL (p=ns).
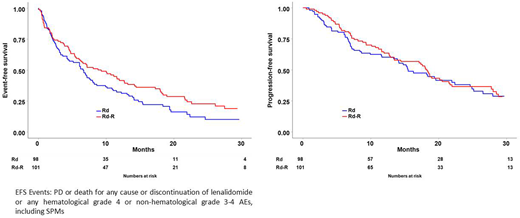
In intention-to-treat analysis, after a median follow-up of 25 months, EFS was 9.3 vs 6.6 months (HR 0.72, 95% CI 0.52-0.99, p=0.04), in Rd-R versus continuous Rd, respectively (Figure 1).
Best response rates were not significantly different between the 2 groups: ≥PR rates were 73% vs 63%, and ≥VGPR rates were 43% vs 35% in the Rd-R vs Rd continuous group, respectively (p=ns).
No difference in progression-free survival (PFS) and overall survival (OS) was observed. Median PFS was 18.3 vs 15.5 months (HR 0.93, 95% CI 0.64-1.34, p=ns) (Figure 1), 18 month-OS was 85% versus 81% (HR 0.73, 95% CI 0.40-1.33, p=ns).
Adverse events accounting for EFS (any hematologic grade 4, non-hematologic grade 3-4) were less frequent in the Rd-R group (30% vs 39%) than in the continuous Rd group (p=ns). The most frequent adverse events were neutropenia, infection and skin reactions (less than 10% in each arm). After 9 treatment cycles, these adverse events were less frequent in Rd-R vs continuous Rd group (3% vs 7%, p=ns).
Lenalidomide dose reduction after 9 treatment cycles was required in 1% of Rd-R patients and 21% of continuous Rd patients (p =0.06). Dexamethasone dose reduction was required in 17% vs 29% of patients, respectively (p=0.06).
CONCLUSION: This is the first prospective randomized phase III trial specifically designed for real-life intermediate-fit NDMM patients. A dose/schedule-adjusted Rd-R treatment was more feasible compared to full dose continuous Rd treatment in elderly intermediate-fit NDMM patients, with no negative impact but rather a comparable outcome. These results confirm the need for an appropriate definition of patient frailty, and pave the way to a frailty-adjusted treatment approach to better balance efficacy and safety in elderly NDMM patients.
Larocca:Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Janssen-Cilag: Honoraria; Amgen: Honoraria. De Paoli:Amgen: Other: Advisory Board; Janssen: Other: Advisory Board; Celgene: Other: Advisory Board; Gilead: Other: Advisory Board. Galli:Celgene: Honoraria; Janssen: Honoraria; Bristol-Myers Squibb: Honoraria; Sigma-Tau: Honoraria. Montefusco:Janssen: Other: Advisory Board; Amgen: Other: Advisory Board; Celgene: Other: Advisory Board. Caravita di Toritto:Johnson & Johnson: Other: Advisory Board, Travel and Accomodation EHA; Amgen: Other: Advisory Board; Bristol-Myers Squibb: Honoraria, Other: Travel and Accomodation EMN; Takeda: Other: Advisory Board; Celgene: Other: Advisory Board, Travel and Accomodation ASH, Research Funding. Giuliani:Celgene Italy: Other: Avisory Board, Research Funding; Takeda Pharmaceutical Co: Research Funding; Janssen Pharmaceutica: Other: Avisory Board, Research Funding. Patriarca:Jazz: Other: Travel, accommodations, expenses; Janssen: Other: Advisory role; Celgene: Other: Advisory Role; Travel, accommodations, expenses; Medac: Other: Travel, accommodations, expenses; MSD Italy: Other: Advisory Role. Offidani:Takeda: Honoraria, Other: Advisory Board; Janssen: Honoraria, Other: Advisory Board; Celgene: Honoraria, Other: Advisory Board; Amgen: Honoraria, Other: Advisory Board; Bristol-Myers Squibb: Honoraria, Other: Advisory Board. Cavo:GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Palumbo:Takeda: Employment. Boccadoro:Bristol-Myers Squibb: Honoraria, Research Funding; AbbVie: Honoraria; Novartis: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Mundipharma: Research Funding. Bringhen:Janssen: Honoraria, Other: Advisory Board; Celgene: Honoraria; Amgen: Honoraria, Other: Advisory Board; Takeda: Consultancy; Bristol-Myers Squibb: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal